2025-03-13

As the pharmaceutical industry increasingly demands higher production efficiency and product quality, the traditional batch production model is gradually revealing its limitations. In contrast, continuous manufacturing has become a focal point in the industry due to its efficiency, flexibility, and resource-saving characteristics. In this transformation, Process Analytical Technology (PAT), as the cornerstone of real-time monitoring and quality control, has emerged as one of the core technologies driving the implementation of continuous manufacturing.
What is PAT?
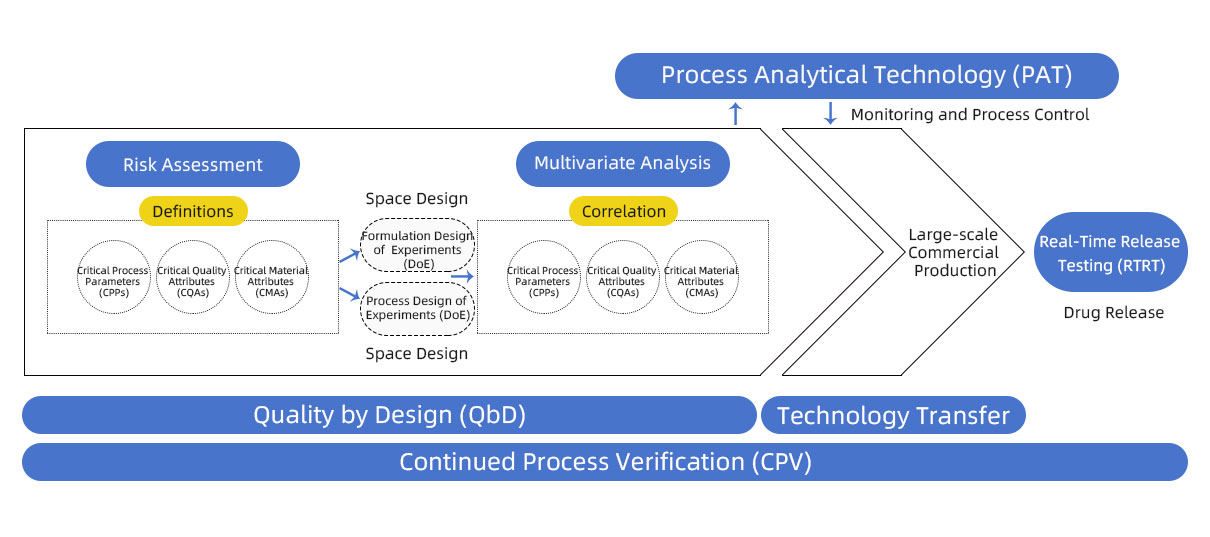
In 2004, the U.S. Food and Drug Administration (FDA) released the "Pharmaceutical cGMPs for the 21st Century—A Risk-Based Approach," officially launching the Process Analytical Technology (PAT) initiative. At its core, PAT aims to realize the concept of "Quality by Design" (QbD) through real-time monitoring, data analysis, and feedback control. The goal is to reduce public health risks in drug manufacturing by innovating process development, production, and quality assurance systems.
PAT focuses on real-time data collection and analysis, emphasizing critical quality attributes (CQA) and critical process parameters (CPP). By integrating sensors, model algorithms, and automated controls, it ensures the stability of quality metrics during production, thereby reducing the risk of batch failures.
In short, PAT is a monitoring and control system based on real-time product quality attributes and process understanding.
PAT typically includes the following technical modules:
The Application of PAT in Continuous Manufacturing
In continuous manufacturing, the application of Process Analytical Technology (PAT) is crucial as it enables real-time monitoring and dynamic control of the production process, ensuring consistent product quality and process stability. Below are the main applications of PAT in continuous manufacturing:
Real-Time Quality Monitoring
Process Optimization and Control
Reduction of Production Interruptions
Support for Quality by Design (QbD)
Data Integration and Intelligent Production
Resource Efficiency and Sustainable Development
The Three Levels of PAT Implementation
Sensing Layer
Utilizes sensors such as online infrared and Raman spectroscopy to collect real-time data on temperature, pressure, and other parameters, addressing the lag associated with traditional offline testing.
Analysis Layer
Employs machine learning or multivariate statistical models to identify data anomalies and predict quality trends. For example, in chemical synthesis reactions, dynamic analysis of reactant concentration changes is used to optimize reaction conditions.
Control Layer
Feeds analysis results back to actuators for automatic parameter adjustment. For instance, in biopharmaceutical fermentation processes, oxygen supply is adjusted in real time to maintain an optimal metabolic environment.
Currently, the most widely used PAT detection technologies are Near-Infrared Spectroscopy (NIR) and online laser particle size analyzers. Figure 2 illustrates common PAT tools and their specific application scenarios in OSD (Oral Solid Dosage) production.
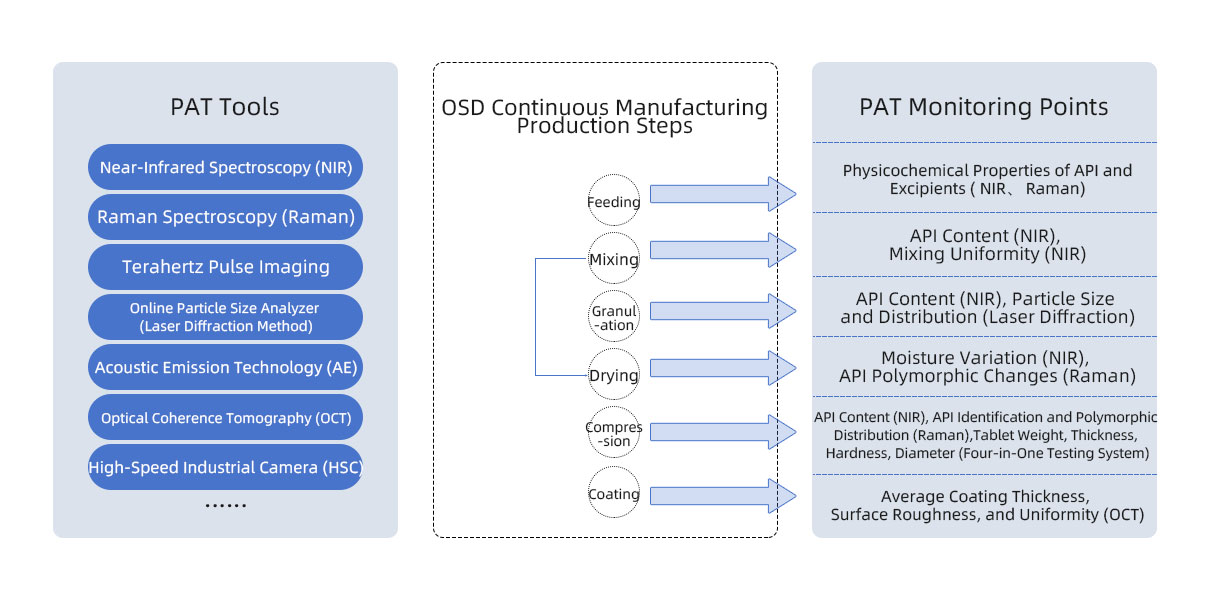
Figure 2. Common PAT Tools and Their Applications in OSD (Oral Solid Dosage) Production.

Figure 3. Physical Examples of Several PAT Tools.
The aforementioned PAT tools can be combined and applied according to practical needs. Meanwhile, with advancements in spectroscopy and measurement technologies, increasingly sophisticated PAT tools are playing an ever more important role in both laboratory research and industrial production.
Key Challenges in the Implementation of PAT
Despite the significant advantages of PAT technology, its large-scale application still faces multiple challenges:
Complexity of Data Integration
Real-time analysis of multi-source heterogeneous data requires robust data infrastructure.
Regulatory and Standards Adaptation
The regulatory framework and standards for PAT are still being refined.
Technical Barriers and Costs
A skilled technical team with in-depth understanding of material properties and chemometrics is required.
Application Cases of PAT
Application of Near-Infrared Spectroscopy (NIR) in a Continuous Direct Compression System
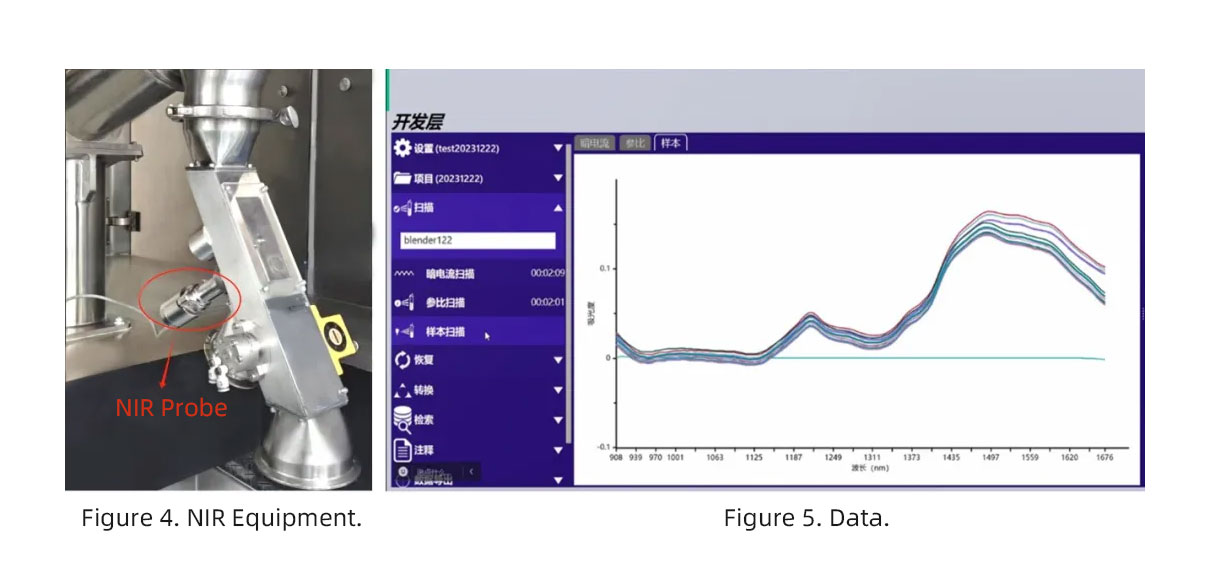
As shown in Figure 4, the NIR device installed at the discharge port of the continuous mixer can monitor the uniformity of API and excipient mixing as well as moisture content in real time during the mixing process. The data can be displayed intuitively, as illustrated in Figure 5.
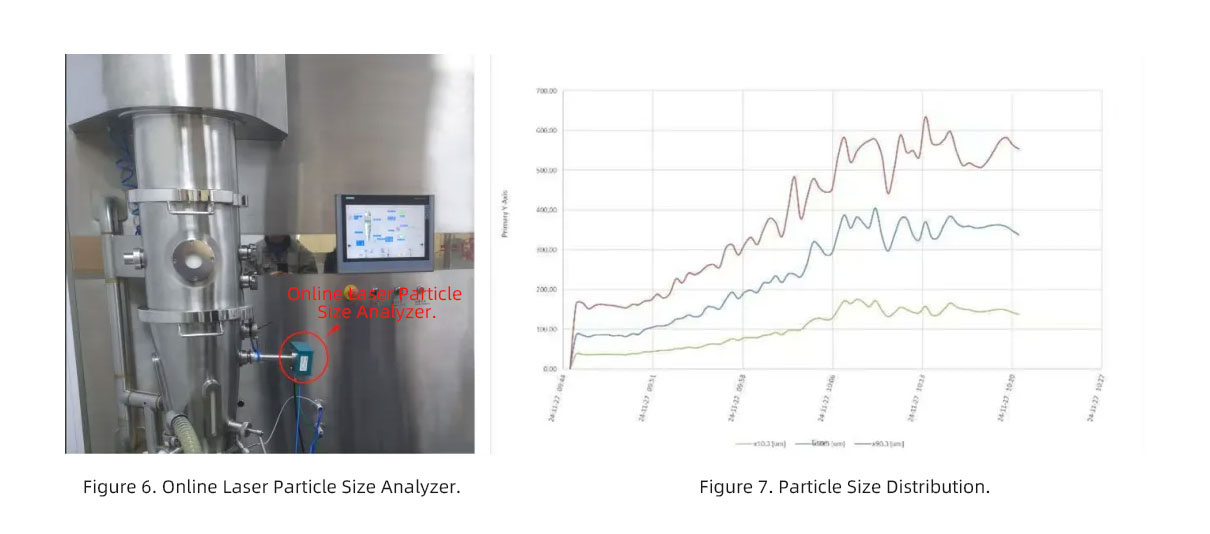
Application of Online Laser Particle Size Analyzer in Fluidized Beds
The online laser particle size analyzer (Figure 6) can monitor the particle size distribution in fluidized beds in real time, providing insights into the real-time status of particles (Figure 7).
The emergence of PAT (Process Analytical Technology) has further driven innovation in drug development, manufacturing, and quality assurance. With the promotion of 21st-century cGMP and the release of the FDA PAT Guidance in 2004, the industry anticipates broader implementation of PAT throughout the drug production process. These initiatives aim to encourage the pharmaceutical industry to leverage technological advancements to enhance product quality and production efficiency. While some may argue that the full implementation of PAT will take time, it is undeniable that PAT has already brought significant transformation and challenges to the pharmaceutical industry.
In the current context of national advocacy for "intelligent manufacturing," the pharmaceutical industry is transitioning from traditional manual decision-making control to more advanced process control. Integrating advanced PAT tools into the manufacturing process, supported by Model Predictive Control (MPC) systems, enables automated process control. The MPC concept involves combining linear process models with PAT online monitoring systems, executing predefined output results based on appropriate control strategies (model-based and non-model-based control).
Wenzhou Jinbang Light Industry Machinery Co., Ltd. is a leading solution provider in the pharmaceutical industry, focusing on promoting the deep integration and innovative application of continuous manufacturing and PAT. During this critical period of transformation and upgrading in the pharmaceutical industry, we recognize the significant value of continuous manufacturing's efficiency and PAT's real-time capabilities in improving product quality, optimizing production processes, and reducing costs. With years of technical expertise and industry experience, we offer customers one-stop solutions ranging from process design and equipment selection to system integration. Our continuous manufacturing systems are equipped with advanced PAT tools, high-performance online sensors, and intelligent data acquisition and processing systems, enabling real-time monitoring of product status and automated control and optimization of production processes.
"Promoting systematic upgrades in pharmaceutical manufacturing capabilities" is one of the five key tasks outlined in the 14th Five-Year Plan. Developing advanced biopharmaceutical manufacturing technologies is a direction strongly advocated by the nation now and in the future. Active Pharmaceutical Ingredients (APIs) are the foundation of the pharmaceutical industry, and crystallization is a critical step in the API production process. The introduction of PAT helps deepen the understanding of API crystallization processes and product quality, enhances control over production processes, and ultimately ensures the quality of APIs. In the future, PAT will undoubtedly see more extensive development and application in the pharmaceutical field.